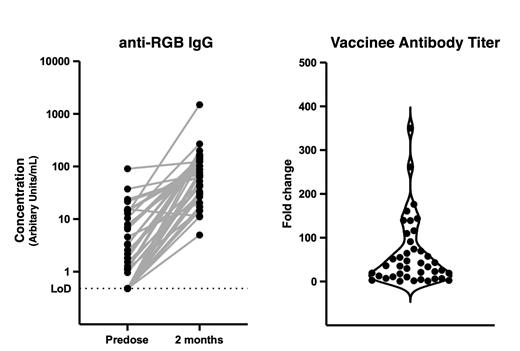
A multi-state, long term follow-up study of individuals with sickle cell disease (SCD) demonstrated an increase in mortality and morbidity in children and adults with COVID. mRNA vaccines are recommended for all SCD patients. Vaccination in individuals with SCD for other infections may be associated with impaired antibody responses. Concern has also been raised about whether mRNA vaccines, through a unique mechanism, may induce disease-related-complications in patients with SCD resulting in a suboptimal immunization rate. The ASH Research Collaborative Clinical Trial Network (ASH RC CTN) performed a mRNA vaccine trial to assess whether antibody responses following COVID vaccination were adequate to induce immunity and whether mRNA vaccination precipitated any toxicity unique to SCD patients. We prospectively studied patients at 8 of the ASH RC CTN sites: Johns Hopkins, Prisma, Duke, UT Southwestern, Medical College of Wisconsin, UCSF, Children's National, and Montefiore. Eligible patients with SCD had not yet received the COVID vaccine. We enrolled 59 patients and 47 received at least one vaccination. All vaccinated patients received the monovalent Pfizer vaccine. Forty-two patients received two vaccinations and provided baseline blood samples prior to vaccination and 2 months after the initial vaccination. For this report, data for 41 paired samples were available for analysis of IgG reactivity against the receptor binding domain (RBD) of the SARS-CoV-2 spike protein. Samples were taken from 7 patients (17%) under 3 years of age, 17 (41%) from older children (3-17 years of age), and 17 (41%) from adults (18-53 years of age). The majority of patients had HbSS (73%) or HbSC (22%), and one of each had either HbS beta 0 (2%) or HbS beta+ (2%). Concurrent therapies included hydroxyurea (59%), voxelotor (17%), crizulizumab (2%), intravenous immunoglobulin (2%), and chronic transfusions (2%). Five had a history of splenectomy (12%). Seventy-five percent of patients received their initial vaccination within 10 days of the baseline visit, and the median time between initial vaccination and post vaccination blood draw was 62 days. One patient experienced a COVID infection 5 days after the baseline visit, but prior to vaccination. This individual later received two vaccinations and submitted blood samples for analysis. Post-vaccination fever/chills, arthralgias, emesis, or local vaccination pain/erythema were reported in 49% of the patients. None reported fever that required inpatient admission. Seven patients (17%) reported a total of 8 episodes of vaso-occlusive pain within 14 days of the 1st vaccination. Of these, three required evaluation and treatment at a medical facility, and one patient was admitted for inpatient care. Antibody responses are shown in the Figure. Fourteen patients (34%) were seronegative at baseline, all with IgG ≤ 0.48 arbitrary units (AU). Twenty-seven patients (66%) were seropositive at baseline with a median IgG = 4.59 AU. Post-vaccination demonstrated a median IgG = 32.2 AU for the initially seronegative patients, and IgG = 102 AU for those initially seropositive. All initially seronegative patients converted to seropositive post vaccination; although post-vaccination IgG levels were lower than in the patients who were initially seropositive (Wilcoxon rank sum test, p-value = 0.0062). Overall, patients with sickle cell disease had side effects similar to those in the general population, and patients with SCD also responded to the COVID mRNA vaccination as expected after 2 months. The 6-month titers will be analyzed in the early fall. To our knowledge, this is the largest mRNA vaccine efficacy and safety trial in individuals with SCD, and it also marks the first evaluation of vaccine safety and antibody response in very young children with SCD.
Disclosures
Anderson:Novo Nordisk: Consultancy; Pfizer: Consultancy, Research Funding, Speakers Bureau; Vertex: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy. Strouse:Agios, Takeda, Disc Medicine: Consultancy, Research Funding. Manwani:Novartis, Pfizer, Novo Nordisk, Editas, GBT: Consultancy. Vichinsky:GBT/Pfizer, Agios Pharmaceuticals: Consultancy, Other: Editor- UpToDate. Field:BEAM: Research Funding; Vifor: Research Funding; Forma: Research Funding; Takeda: Research Funding. Lanzkron:Teva Pharmaceutical Industries: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; Novo Nordisk: Consultancy; Bluebird Bio: Consultancy; Novartis: Research Funding; Shire: Research Funding; Imara/Enliven Therapeutics: Research Funding; Global Blood Therapeutics: Research Funding. Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal